
Discovery of the Bohr-van Leeuwen Theorem Likewise, through these concepts, he was able to obtain a notion of how matter is capable of absorbing and emitting light from its most imperceptible internal structures. With these applications, Bohr was able to determine the movements of the electrons around the atomic nucleus, as well as the changes in its properties. What led to the atomic model of Bohr to be considered revolutionary, was the method that it used to obtain it: the application of theories of quantum physics and its interrelation with the atomic phenomena. Bohr introduced the atomic structure as a small solar system. To obtain this atomic model, Bohr applied Max Planck's quantum theory to the atomic model developed by Rutherford, resulting in the model that earned him the Nobel Prize. The number of electrons present in the outer orbit of the nucleus determines the properties of the physical element. It was the first to exhibit the atom as a positively charged nucleus and surrounded by orbiting electrons.īohr was able to discover the mechanism of internal functioning of an atom: the electrons are able to orbit independently around the nucleus. The atomic model discovered by Niels Bohr is considered one of his greatest contributions to the world of physics and the sciences in general.

Main contributions of Niels Bohrīohr and Albert Einstein Model and structure of the atom Niels Bohr started from the discoveries made by Rutherford to continue developing them until he could print his own imprint.īohr came to have a family of more than six children He became tutor to other scientific eminences such as Werner Heisenberg Was president of the Royal Danish Academy of Sciences, as well as member of other scientific academies around the world. Thompson and Ernest Rutherford, who urged him to continue his research in the atomic area.īohr's interest in atomic structure led him to move between universities until he found one that would give him the space to develop his research under his own terms. He worked with other renowned scientists and Nobel Prize winners, such as J.J.
#NIELS BOHR ATOMIC THEORY FREE#
The electron in an atom is more stable than a free electron.Raised and educated in European lands, in the most prestigious English universities, Niels Bohr was also a renowned researcher and curious philosopher. It corresponds to an ionised atom where the electron and the nucleus are infinitely separated. It is the maximum energy that an electron can possess in an atom. These shells are numbered as one, two, three, four or termed as \(\rm \), the energy becomes zero.
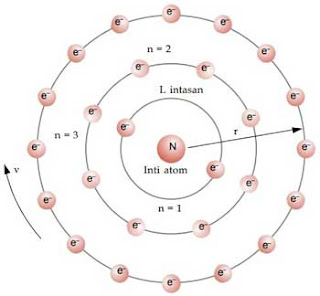
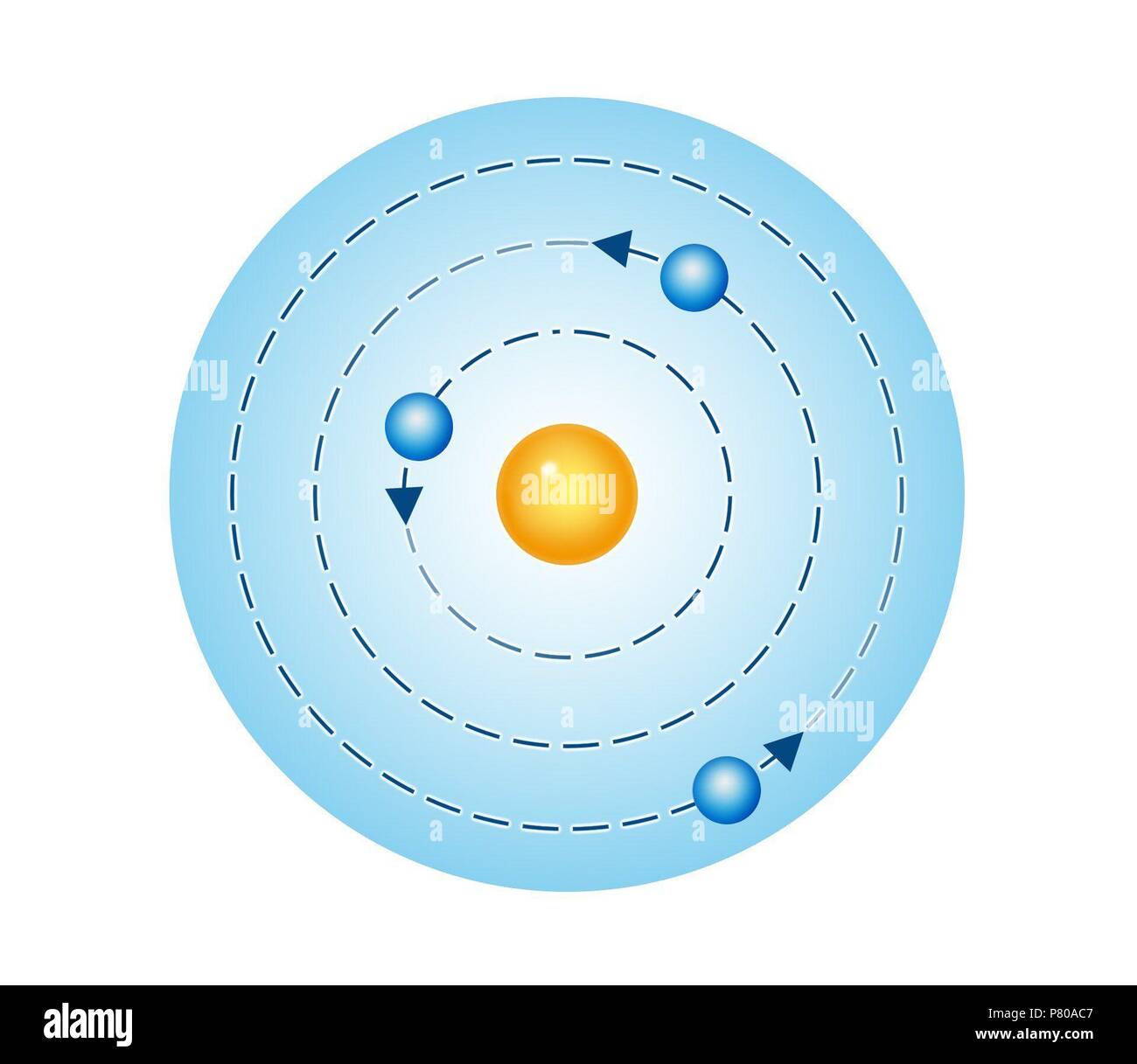
It needed slight modifications that were made by a legendary scientist named Neil’s Bohr.īohr recognised that the lines in an atomic spectrum are related to the arrangement of electrons in that atom. For decades, many questions had been asked about atomic characteristics. In 1913, after returning to Copenhagen, he began publishing his theory of the simplest atom, hydrogen, based on the planetary model of the atom. Bohr became convinced of its validity and spent part of 1912 at Rutherford’s laboratory.

The great Danish physicist Niels Bohr (1885–1962) made immediate use of Rutherford’s planetary model of the atom. Rutherford’s Atom Model was undoubtedly a breakthrough in atomic studies.


 0 kommentar(er)
0 kommentar(er)
